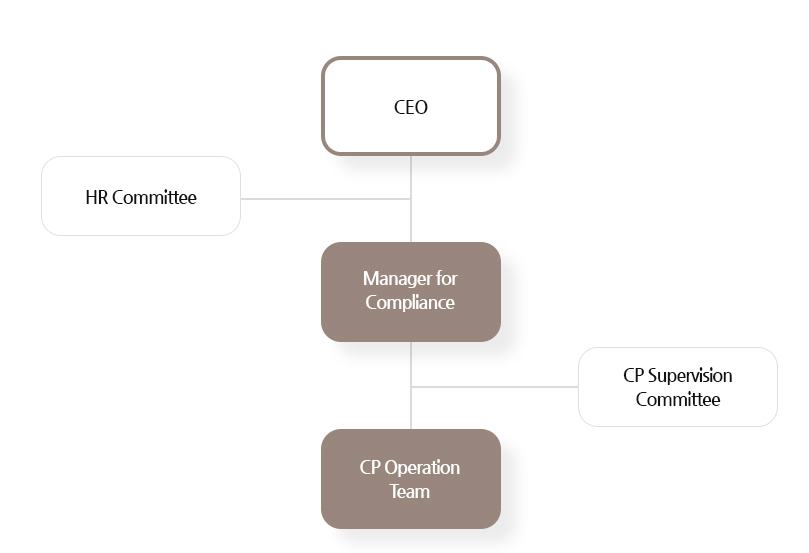

CP Organization Chart
CP Organization Chart and its Roles


| Division | Roles |
|---|---|
| Manager for compliance | Overall responsibility for compliance program |
| Operational management of CP report / consultation | |
| Deliberation on punishment against CP violators and presentation on HR committee | |
| Report operational matters of compliance program to executives and board of director | |
| CP Supervision Committee | Consultation on issues related to in-house CP |
| Serve a role as a bridge of each job related to CP | |
| Review CP risks in advance during job performance | |
| CP Operation Team | Overall responsibility of Compliance Program operation system |
| Monitoring (In advance / Post / In the site) | |
| Compliance Program training for employees | |
| Conduction of compliance conference |
Operational status of CP






136, Changgyeonggung-ro, Jongno-gu, Seoul, Republic of Korea (03127)
Copyright (C) 2022 by Boryung Corporation. All Rights Reserved.